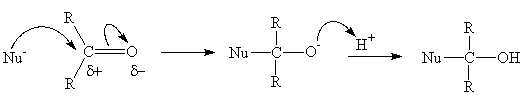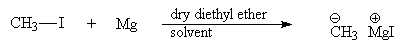ALDEHYDES AND KETONES
Reference: McMurry Ch 9 George et al Ch 2.6
Structure and bonding
- Contain a carbonyl group, C=O
- Aldehydes have at least one H attached to the carbonyl group, ketones have two carbon groups attached to the carbonyl group
- Carbon of the carbonyl group is sp2 hybridised

- Aldehydes and ketones strongly absorb radiation around ~ 1700 cm-1 in the infrared region
Nomenclature
Aldehydes
- The longest chain containing the CHO group gives the stem; ending –al
- If substituents are present, start the numbering from the aldehyde group - C1
Ketones
- The longest chain containing the carbonyl group gives the stem; ending –one
- If substituents are present number from the end of the chain so the carbonyl group has the lowest possible number
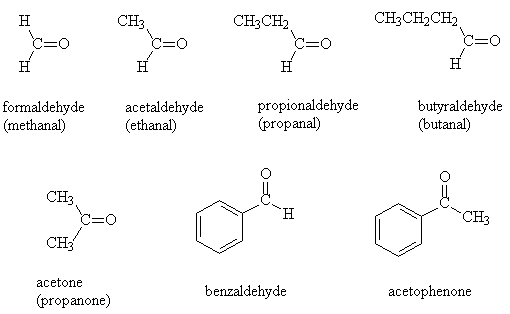
- There are non-systematic names for the common aldehydes and ketones
Reactions of Aldehydes and Ketones
With the exception of oxidation of aldehydes, the reactions of aldehydes and ketones is dominated by nucleophilic addition.
1. Oxidation of aldehydes
- Aldehydes (but not ketones) may be oxidised to carboxylic acids with Cr2O72- / H+
Example:

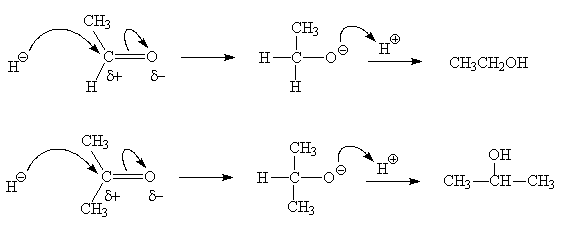
2. Nucleophilic addition
- The double bond of the carbonyl group undergoes an addition reaction
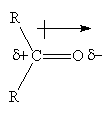
- The polarity of the C=O bond results in the addition of a nucleophile (Nu-) to the carbon atom, breaking of the double bond and addition of H+ to the oxygen is always the second step and results in an alcohol
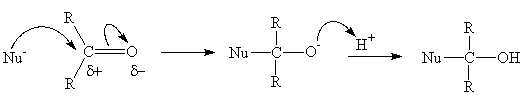
- Common nucleophiles include the Grignard reagent (RMgX), hydride ion (H- from LiAlH4 or NaBH4)
In summary

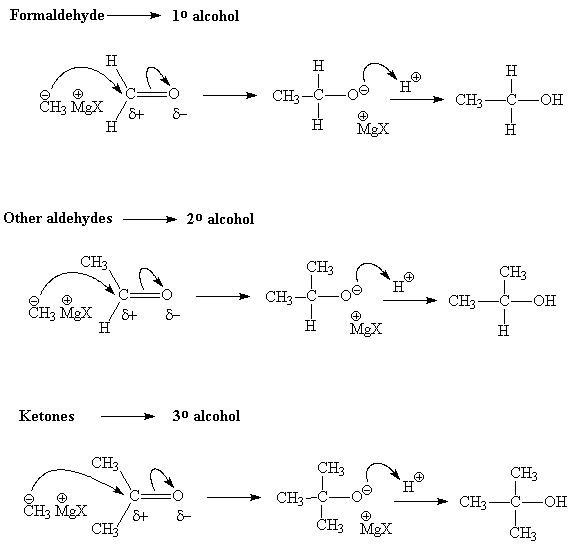
Examples: Grignard reaction
Recap – generation of a Grignard reagent from an alkyl halide and magnesium in dry diethyl ether solvent
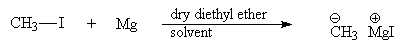
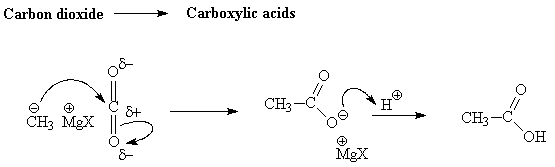
Grignard reagents also react with carbon dioxide to generate carboxylic acids after addition of aqueous H+
Reduction
Reduction of the non-polar C=C or Cº C bonds in alkenes and alkynes respectively, can be accomplished with (non-polar) hydrogen gas, H2. The polar C=O group requires a polar source of hydrogen: H- / H+. The hydride ion (H-) is supplied by a metal hydride, LiAlH4 or NaBH4 and the H+ by an aqueous acid "quench".
- Aldehydes are reduced to 1° alcohols and ketones to 2° alcohols
Addition of alcohols
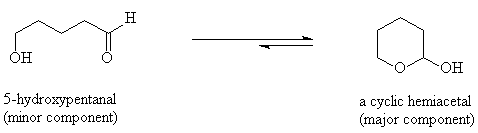
Alcohols add to aldehydes and to ketones to form hemiacetals. When an aldehyde or ketone is dissolved in an alcohol solution, the free carbonyl compound is in equilibrium with its hemiacetal derivative.

Typically the equilibrium strongly favours the free aldehyde or ketone except when an aldehyde or ketone can react intramolecularly (i.e. within the same molecule). If a 5- or 6-membered ring is formed then the cyclic hemiacetal is the major species in the equilibrium.
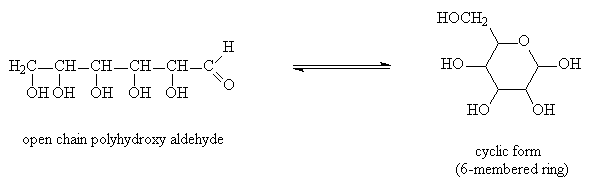
Carbohydrates
Carbohydrates, or sugars, have the general formula Cn(H2O)n
They are usually composed of a linear (unbranched) chain of C atoms with several alcohol functional groups and an aldehyde or ketone group
In solution carbohydrates exist as an equilibrium mixture of open chain polyhydroxy-aldehyde and cyclic hemiacetal compounds.
Example: Glucose
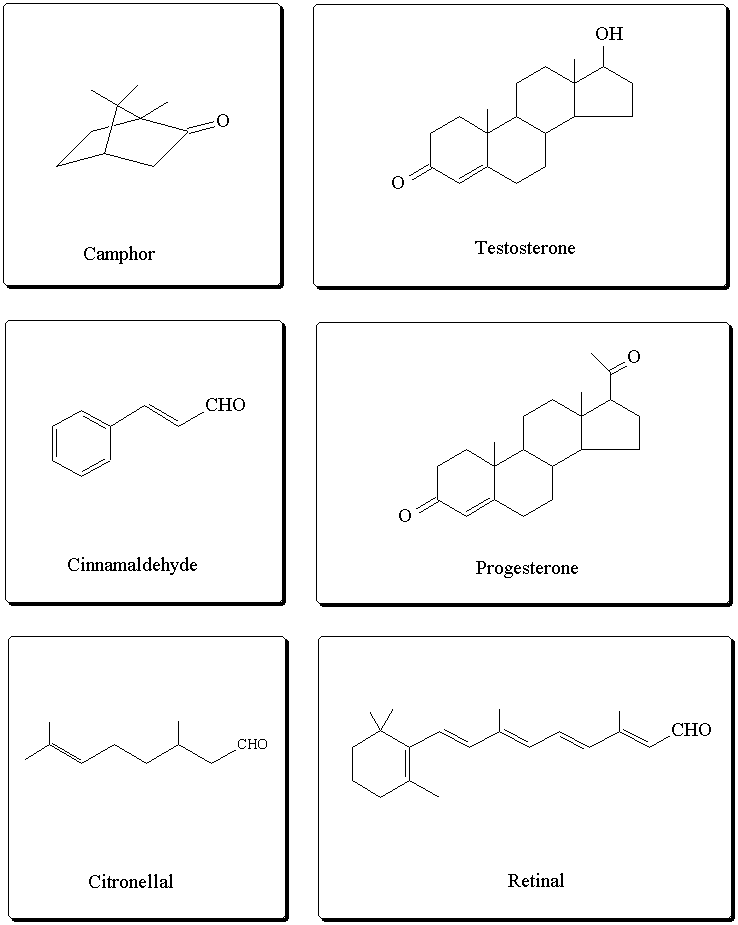
Some biologically active compounds containing a
carbonyl functional group:
Questions on Aldehydes and Ketones
Return to the Index