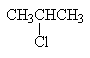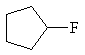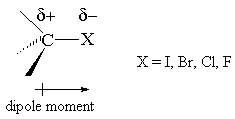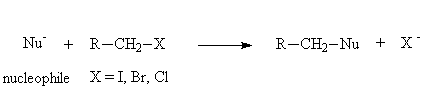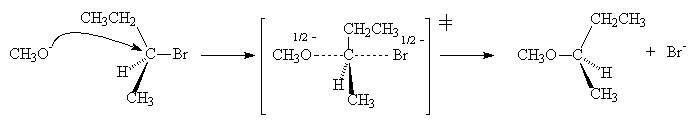ORGANIC HALOGEN COMPOUNDS
Reference: McMurry Ch 7 George et al Ch 2.1
Nomenclature
Rules for naming halogen compounds follow the guidelines already given for hydrocarbons, in summary
- The number of carbon atoms in the longest carbon chain (or one containing multiple bonds if present) gives the stem
- Name the halogen atoms as substituents: fluoro, chloro, bromo or iodo
- If more than one halogen atom present, order names alphabetically
- Use numbers and di-, tri- etc as appropriate
- Organic halogen compounds may be classified as primary (1° ), secondary (2° ), tertiary (3° ) or aryl halide depending on whether the carbon atom bearing the halogen is attached to 1 other carbon group, 2 other carbon groups, 3 other carbon groups or an aromatic ring respectively.
Examples:
|
Structure
|
Name |
Class |
|
CH3CH2Br
|
bromoethane |
primary alkyl halide |
|
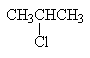
|
2-chloropropane
|
secondary alkyl halide |
|
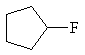
|
fluorocyclopentane |
secondary alkyl halide |
|
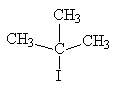
|
2-iodo-2-methylpropane
(also called tertiary-butyl iodide) |
tertiary alkyl halide |
|
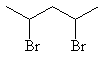 |
2,4-dibromopentane |
two secondary alkyl halides |
|

|
4-chlorotoluene |
aryl halide |
- A few special names include chloroform (CHCl3) and carbon tetrachloride (CCl4)
Structure and properties
- Alkyl halide compounds are mostly dense liquids and solids that are insoluble in water.
- The halogens are all more electronegative than carbon and this makes the carbon-halogen bond a polar bond with a slight positive charge (d +) residing on the carbon end of the bond and a slight negative charge (d-) on the halogen end.
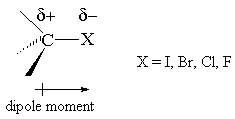
- The carbon-halogen bond strength decreases in the order C-F > C-Cl > C-Br > C-I
- Alkyl fluorides tend to be less reactive than other alkyl halides, mainly due to the higher strength of the C-F bond.
Reactions of Alkyl Halides
The reactivity of alkyl halides is dominated by the attack of nucleophiles at the carbon atom that bears the halogen atom. This results in a nucleophilic substitution reaction. There is a wide range of nucleophiles that may be employed. Simple aryl halides do not tend to undergo nucleophilic substitution as the attacking nucleophile is repelled by the high electron density of the aromatic ring.
1. Nucleophilic substitution
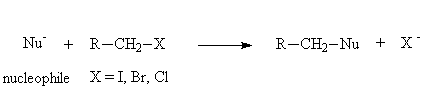
Examples:
|
Nucleophile (Nu–) |
+ |
CH3I |
® |
Product |
+ I– |
Product Class |
|
HO– (hydroxide) |
+ |
" |
® |
CH3-OH |
+ I– |
alcohol |
|
R'O– (alkoxide) |
+ |
" |
® |
CH3-O-R' |
+ I– |
ether |
|
NºC– (cyanide) |
+ |
" |
® |
CH3-CºN |
+ I– |
nitrile |
|
R'-CºC– (alkynide) |
+ |
" |
® |
CH3-CºC-R' |
+ I– |
longer alkyne |
|
:NH2–(amide) |
+ |
" |
® |
CH3-NH2 |
+ I– |
amine |
|
:NR3 (tertiary amine) |
+ |
" |
® |
CH3NR3+Y– |
+ I– |
tetraalkylammonium salt |
The mechanism of nucleophilic substitution for primary and secondary alkyl halides is abbreviated to SN2, short for Substitution, Nucleophilic, second order. It is a bimolecular reaction.
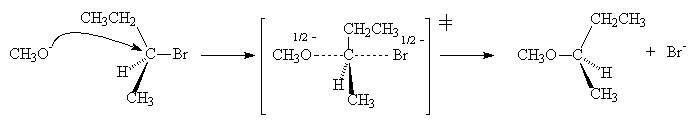
As the number of substituents around the carbon centre undergoing reaction increases, the substituents block the approach of the incoming nucleophile and consequently an SN2 mechanism becomes less favourable.
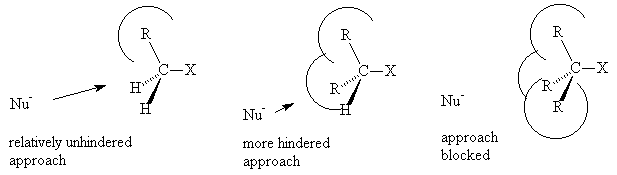
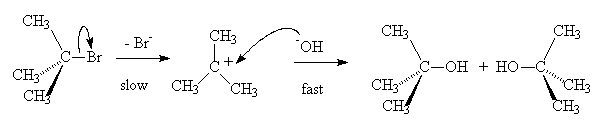
In the case of a tertiary alkyl halide, loss of the halide occurs first to give a carbocation which then reacts with the nucleophile. This is called a SN1 reaction (Substitution, Nucleophilic, first order).
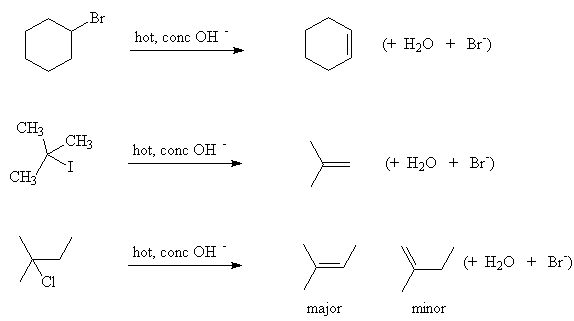
2. Elimination
Alkyl halides can also undergo an elimination reaction to form an alkene. Effectively HX is eliminated.
- This requires a concentrated strong base (e.g. KOH in alcohol solvent) and heat
- The halide is removed along with a hydrogen from the adjacent carbon atom
- Tertiary alkyl halides undergo an elimination reaction more easily than secondary alkyl halides which, in turn, are more reactive than primary alkyl halides
- Where there is a choice of hydrogens that can be eliminated, the one that results in the most substituted alkene is removed
Examples:
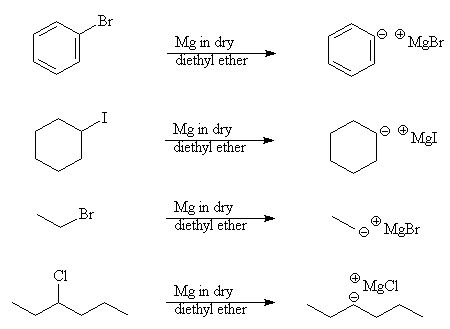
3. Formation of a Grignard reagent
- Both alkyl and aryl halides react with magnesium in dry ether solvent to form a Grignard reagent
- This is an organometallic compound (contains carbon bonded to a metal) and is a useful intermediate in the synthesis of alcohols and carboxylic acids
Example:
Some biologically active compounds containing
halide atoms:

Questions on Organic Halogen Compounds
Return to the Index