Biomimicry and polymer surfaces recreate nature for industry use
Surfaces in nature can be anything from super water-repellent to super slippery, or able to pull water from the air. Now Dr Chiara Neto and her team are working on recreating those surfaces to solve human problems.
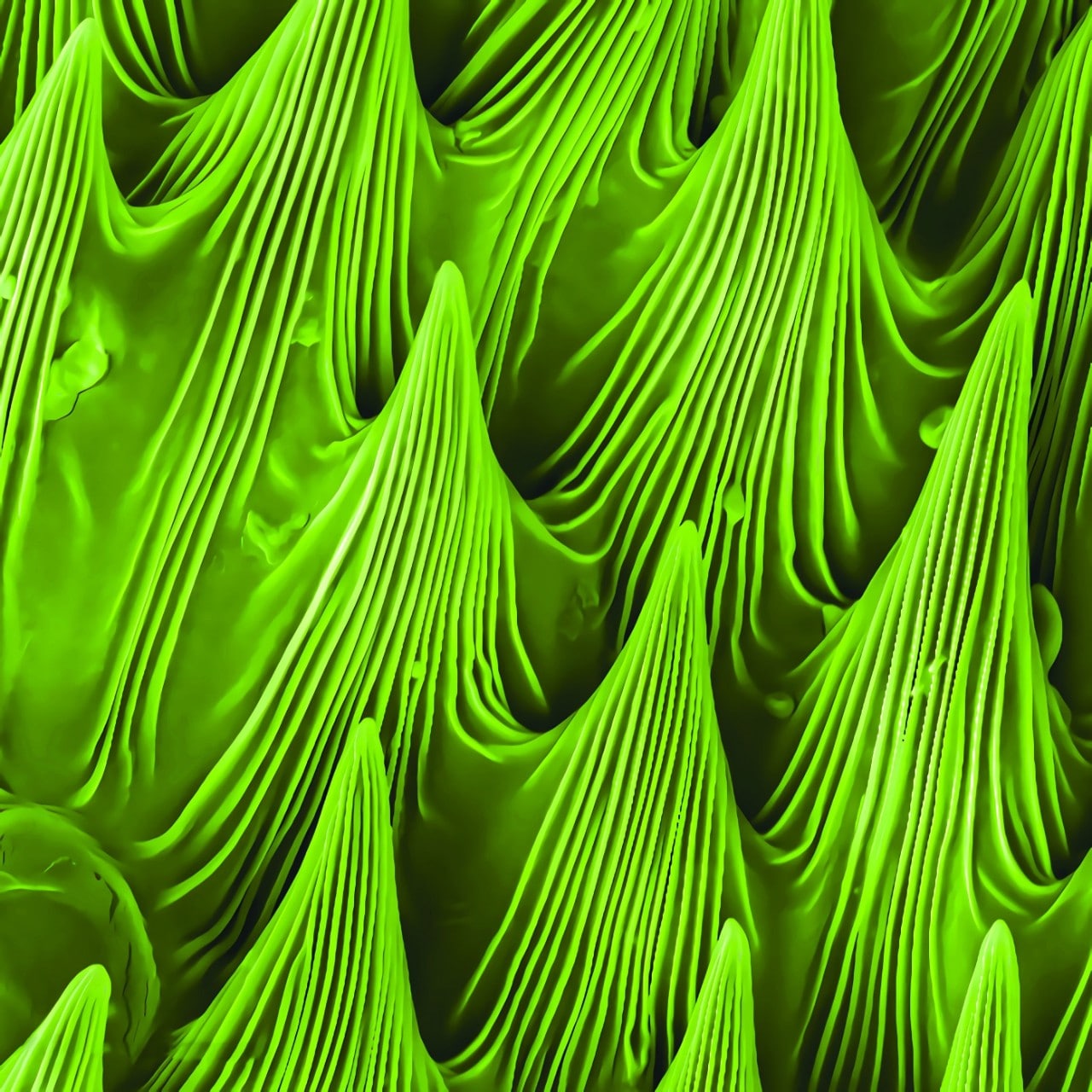
The surface of the Nepenthes pitcher plant.
The carnivorous pitcher plant is named for its built-in pitcher, filled with digestive juices for dissolving any insect that slides into it. The plant’s slippery inner coating means even the most sure-footed insect is likely to succumb to the pitcher.
It’s an unlikely prompt for a technology that could save the shipping industry billions of dollars a year, but one that presented an opportunity for the University’s Neto Research Group. The group has used the pitcher plant as a starting point to create a coating for the submerged surfaces of boats, a coating too slippery for barnacles and other ocean life to attach.
Minute pinholes in a polymer film at the start of ‘dewetting’, as the film retracts from the substrate.
This is a research area called biomimicry: the replication of traits occurring in nature to create new materials and solve engineering problems. The concept of biomimicry isn’t new; more than 500 years ago, Leonardo da Vinci famously studied bird flight to draw up plans for flying machines. Elsewhere in the 1950s, Swiss engineer George de Mestral developed Velcro after finding burrs stuck to his socks.
Biomimicry captured the imagination of Associate Professor Chiara Neto more than 15 years ago, when she was doing postdoctoral research in Germany. There, she became interested in superhydrophobic surfaces, those which naturally repel water and are useful, for example, in corrosive environments. This superhydrophobic effect is also called the lotus effect because its water repelling properties are like those of a lotus leaf.

Associate Professor Chiara Neto in front of a scanning electron microscope (SEM) micrograph of a wrinkled polymer surface. She is holding the same polymer surface with a scratch in it.
“Seeing a droplet roll off a lotus leaf, leaving nothing behind, is an image anybody can appreciate,” says Neto. But the structure of that leaf cannot be fully understood, and potentially replicated, without close examination. To that end, Neto and her team study how liquids and solid surfaces interact to the scale of the nanometre (one nanometre [nm] is a billionth of a metre; a human hair is about 75,000 nm wide).
When SAM visits Neto, who is an Australian Research Council (ARC) Future Fellow, she shows off her domain – a laboratory in the School of Chemistry on Eastern Avenue – with pride. Sun shines into the laboratory and there is a constant thrum, like a chorus of refrigerators. Researchers work with quiet intent at machines unknown to most - spectrometers, atomic force microscopes, sonicators – that allow them to look at natural materials which have evolved over thousands of years so that their structures might be copied and applied in new ways.
“A lot of plants are very effective, but they’re only effective for a short time because plants don’t live long,” says Neto. “What we’re trying to do is to translate a plant’s mechanisms to materials that will last a lot longer.”
This is all a long way from Florence, where Neto grew up and, like her parents, studied chemistry.
“For me chemistry was a familiar and friendly sort of topic,” she says. She is quiet and serious, softly spoken but brimming with enthusiasm. “I didn’t feel threatened by it.”

Applying crystals of stearic acid produces highly water-repellent surfaces.
The University of Florence cultivated her interest in intermolecular forces which determine how molecules interact within certain structures. Their close examination requires atomic force microscopes which “feel” the surface with a microscopic probe. This allows scientists to investigate molecular activity on the nanoscale.
Neto’s first major breakthrough came while completing her PhD at the Australian National University. It challenged what’s called the no-slip boundary condition, essentially rewriting accepted knowledge on how moving liquids and solid surfaces behave when in contact with each other. This is important knowledge when considering drag, which affects things like boats travelling through water, with implications for speed and fuel efficiency.
Though hotly contested at the time, Neto’s finding eventually gained acceptance and remains one of her most cited pieces of research.
A lectureship brought Neto to the University of Sydney in 2007. Here, she has bedded down her research and created the Neto Research Group, a group of 11 doctoral and postdoctoral researchers who share her research interests.
This is the group that was inspired by the pitcher plant to create an anti-fouling surface for ships. The accumulation of unwanted marine life on the undersides of boats increases a boat’s drag and can push fuel costs by up to 40 per cent. It’s also a biohazard that introduces invasive organisms to marine environments.

Interference fringes causing colours on a thin polymer film, as often seen on soap bubbles.
For forty years, the shipping industry-controlled fouling with paint containing the organic compound, tributyltin. But this was banned in 2003 because of its toxicity, which contaminated ocean wildlife and caused the closure of shellfish farms. The shipping industry is now desperate for an alternative.
In searching for solutions, Neto’s research group developed Teflon wrinkles which were extremely repellent to water and able to reduce drag. With inspiration from the pitcher plant, the researchers were able to go one better and make the wrinkles resistant to unwanted organisms.
The newly developed surface was put to the test in Sydney Harbour, tied to the shark nets at Watson’s Bay for seven weeks.
“We found that very little in terms of algae and larger barnacles and organisms attach to the surface,” says Neto. The few that do attach can be easily removed, so the surface is antifouling and foul-releasing.”
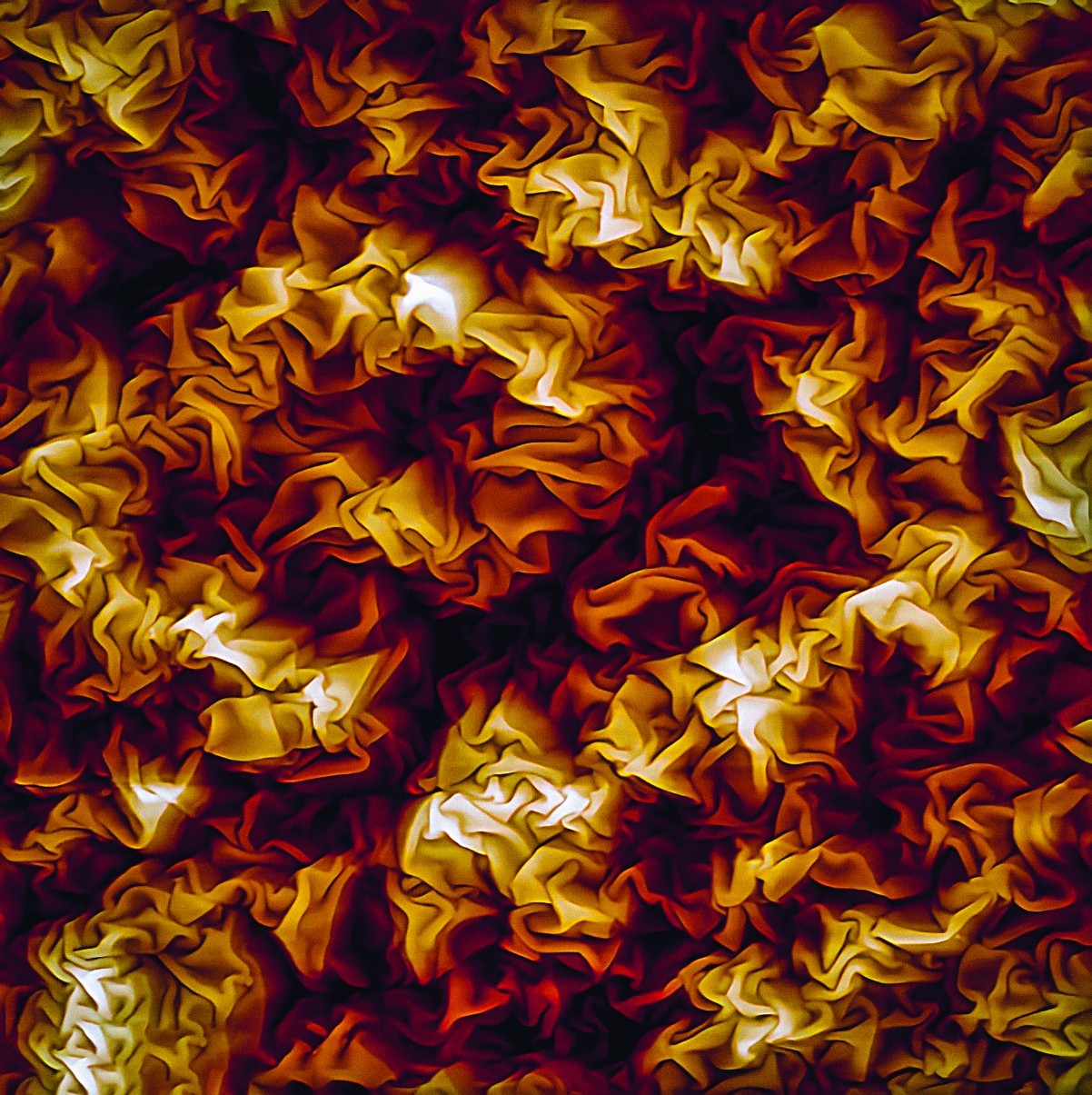
Polymer films can wrinkle in various ways so they’re used to create new and useful surfaces.
Neto is now working with a Sydney-based enterprise to translate this discovery research into a commercial product that can be manufactured at scale.
It has been a long haul for Neto to get from her PhD thesis, which established her academic credibility, to the development of technology that can solve pressing engineering problems. But it has allowed her now to split her time between continuing her fundamental experimental research and the more practical applied research.
Microstructure of a lotus leaf, has a waxy coating and is super water‑repellent.
She’s also encouraging her own children to explore science at a fundamental level.
“My six-year-old is obsessed with experiments. He’s always getting a jar from the under the sink and mixing stuff – powders, liquids, whatever he can find – to see what happens.”
“There’s not much control in the experiments,” she says, breaking into a broad laugh. “But the idea is there.”
Written by Jocelyn Prasad
Photography by Stefanie Zingsheim
