
Researchers explore new synthetic gel for eye injury
The cornea is the clear window that covers the front of the eye. When it is damaged corneal transplantation is required, however, this relies on donor human cornea of which there is a severe worldwide shortage.
University of Sydney researchers are part of the international team who have developed and carried out preclinical testing of a synthetic, biocompatible and adhesive liquid that aims to mend damaged cornea. The new clear gel, called LiQD Cornea, could potentially remove the need for transplants in many cases.
The study is published in the international journal Science Advances.
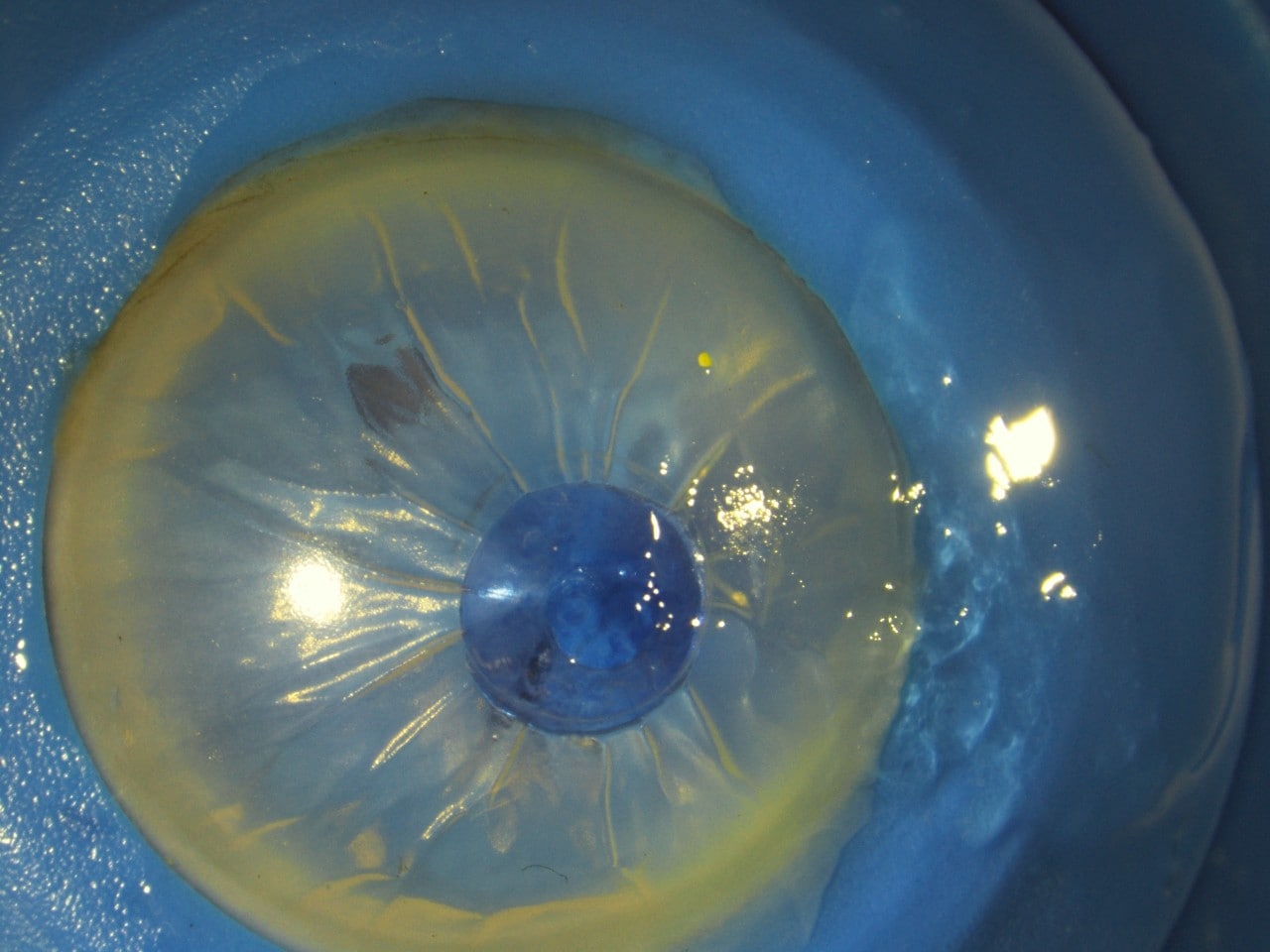
Appearance of the glue on application in a human eye donated for research purposes. A small section is stained blue for visualisation, while another section (far right) shows the glue without stain.
About 4,300 Australian’s suffer corneal injury each year and the figures are much higher in developing countries where agricultural accidents are a common cause.
“While we are more fortunate in Australia, only 1 in every 70 patients worldwide who need a corneal transplant get it,” said co-author Associate Professor Chameen Samarawickrama from the University of Sydney's Faculty of Medicine and Health and The Westmead Institute for Medical Research.
Currently, damage to the cornea from physical trauma or infection, for example from a work-place injury or virus in the eye, is treated through sealing with a type of tissue glue (cyanoacrylate glue) from the superglue family. However, the glue is toxic and without follow-up corneal transplantation, there will still be sight loss.
Associate Professor Samarawickrama collaborated with colleagues at the University of Montreal (Canada) and Linkoping University (Sweden) on the conception and manufacturing of LiQD Cornea. He and post doctoral researcher Damien Hunter carried out a trial with rabbits, one of the two preclinical trials detailed in the paper, finding LiQD Cornea worked very effectively on smaller injuries.

“It acts very much like a dental filling,” said Associate Professor Samarawickrama, associate professor in clinical ophthalmology and eye health in the Faculty of Medicine and Health.
“It comes out as a liquid and sets in a gel form, stays on the eye comfortably, doesn’t scar and remains see-through or translucent, countering many of the limitations of the tissue glue currently used. Our early studies also suggest it allows the eye to continue to regenerate.”
Due to the synthetic nature of the material, risks associated with immune rejection and disease transmission are also reduced in comparison to natural products.
While further preclincal studies are needed to ensure safety and efficacy before progressing to human trials, Associate Professor Samarawickrama said the results are encouraging noting that the cornea is avascular (no blood vessels through it) in all mammals increasing the likelihood of similar outcomes in humans.
“If successful LiQD Cornea could be applied outside the operating room setting meaning there is immense potential to reduce health care costs and increase patient access,” he said.
Declaration: Selected international authors declare competing interests in regards to the invention and patenting of materials related to this study. View the full paper for details. Ethics approval was obtained and all guidelines and legislation governing animal studies were strictly adhered to.