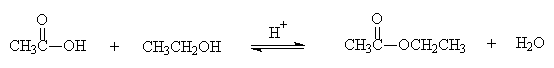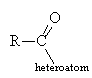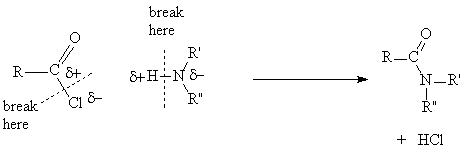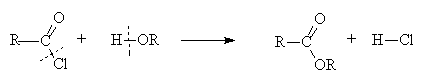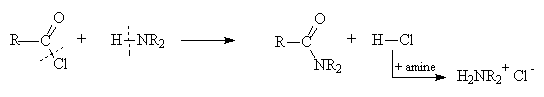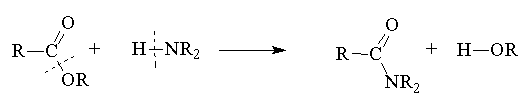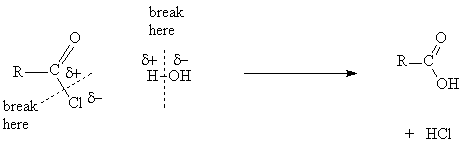CARBOXYLIC ACIDS
Reference: McMurry Ch 10 George et al Ch 2.7
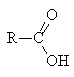
Carboxylic acids contain the functional group:
Nomenclature
- The longest chain containing the COOH group gives the stem; ending –oic acid
- If substituents are present, start the numbering from the carboxyl group - C1
- The conjugate bases are named by replacing the –oic acid ending with –oate
- Some simple carboxylic acids have non-systematic names
Examples
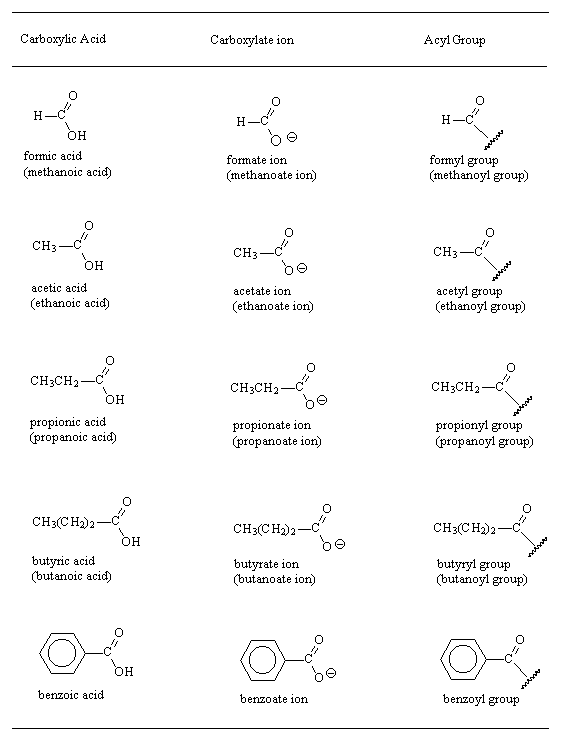
- Carboxylic acids show a strong absorption around ~ 1700 cm-1 (C=O) and a very broad absorption around ~ 3000 cm-1 (O-H) in the infrared region
Reactions of Carboxylic Acids
1. Acid – Base Reaction
- Carboxylic acids are weak acids with pKa values ~ 4-5.
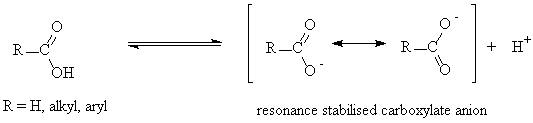
- The carboxylate ion is a resonance stabilised carbanion.
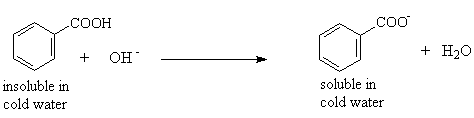
- Carboxylic acids react with dilute aqueous sodium hydroxide solution to form the water soluble carboxylate ion
Example:
2. Esterification
- A carboxylic acid and an alcohol will form an ester and water
- The reaction is an equilibrium
- A catalyst, usually H+, is required
Example:
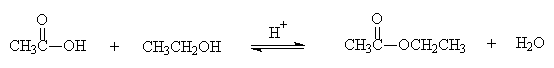
Some biologically active compounds containing the
carboxylic acid functional group:

Questions on Carboxylic Acid Nomenclature
Return to the Index
CARBOXYLIC ACID DERIVATIVES
Reference: McMurry Ch 10 George et al Ch 2.7
- Carboxylic acid derivatives all have an acyl group, attached to a heteroatom (O, N, halogen).
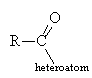
- Three classes dealt with here: acid chlorides, esters and amides (in the lab another derivative, an acid anhydride, is used for convenience)
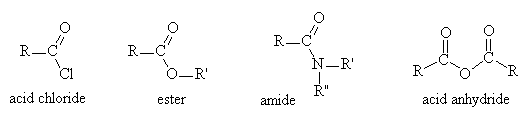
Reactions of Carboxylic Acid Derivatives
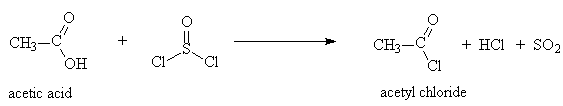
1. Formation of acid chlorides
- Acid chlorides are formed by treatment of a carboxylic acid with thionyl chloride, SOCl2
Example:
2. Interconversion of derivatives
- A more reactive derivative may be converted into a less reactive one
- Acid chlorides are more reactive than esters which are more reactive than amides
- In the outcome of all reactions of acid derivative the bond between the acyl group and the heteroatom breaks and, in the reagent, the bond between the hydrogen and heteroatom breaks. The polarity of the fragments can be used to decide what the final products are.
Example:
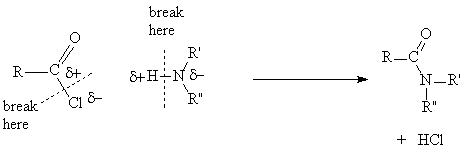
Acid chloride + alcohol ® ester + HCl
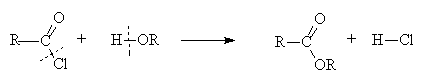
Acid chloride + amine ® amide + HCl
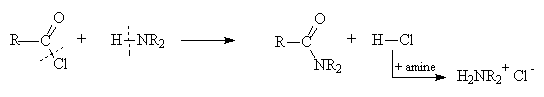
Ester + amine ® amide + alcohol
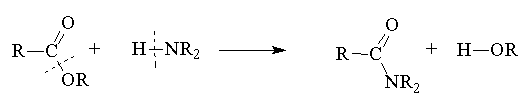
3. Hydrolysis of derivatives
- All derivatives hydrolyse with water to form the carboxylic acid
- The ease of hydrolysis depends on the stability of the derivative: acid chlorides hydrolyse in water at pH 7; esters require a H+ or OH- catalyst and heat to hydrolyse; amides require prolonged heating with more concentrated H+ or OH-
- The reaction is directly analogous to the interconversion of derivatives
Example:
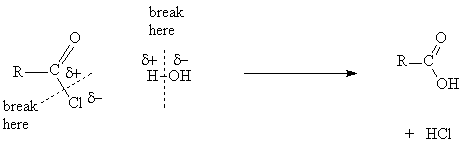
- Hydrolysis under basic conditions results in formation of a carboxylate ion and hydrolysis of amides under acid conditions results in formation of an ammonium ion
|
Derivative |
Relative reactivity |
Conditions of hydrolysis |
Products of acid hydrolysis |
Products of base hydrolysis |
|
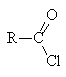
|
high
low
|
H2O |
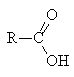 |
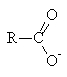 |
|
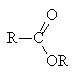
|
H+ /H2O/heat
or
OH- /H2O/heat |

+ ROH |
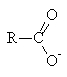 + ROH + ROH |
|
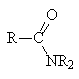
|
H+ /H2O/heat
or
OH- /H2O/heat |

+ R2NH2+ |
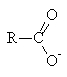 + R2NH + R2NH |
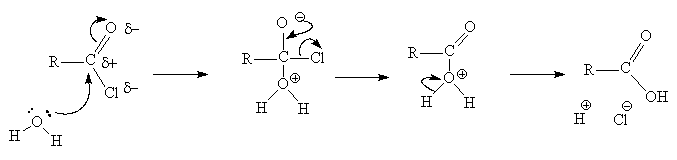
Mechanism of reaction
- The mechanism is the same for all the acid derivative interconversions and hydrolysis reactions
- It is a nucleophilic substitution reaction (addition + elimination = substitution)
Amino Acids:
Proteins are important natural amides. They are polymeric materials formed from amino acids. Amino acids have both an amine functional group and a carboxylic acid functional group in the same molecule and proteins are formed by linking the amine group from one molecule with the carboxylic acid group of another to form an amide bond.
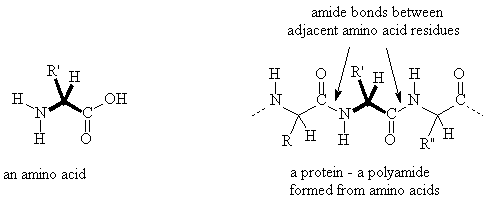
Hydrolysis of a protein is typically achieved by treating it with boiling 6M hydrochloric acid. This hydrolyses all of the amide bonds and releases all of the amino acids which make up the protein.
Proteins generally consist of hundreds of amino acid residues linked together to form a single molecule with a molecular weight of >100,000.
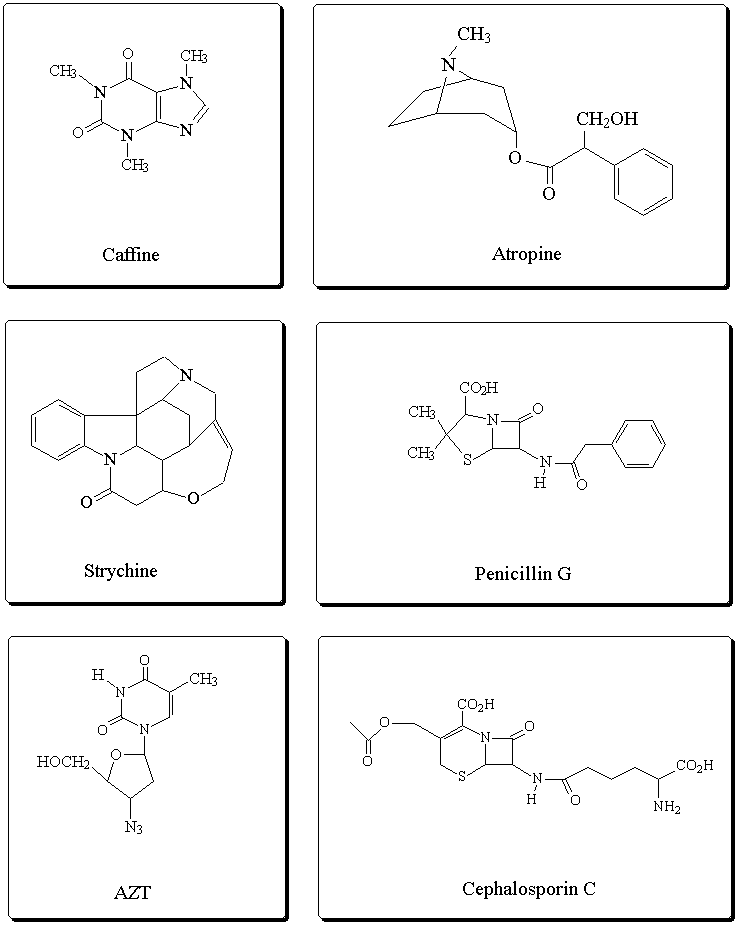
Some biologically active compounds containing the
carboxylic acid derivatives:
Questions on Carboxylic Acid Derivatives
Return to the Index