
SPECTROSCOPY & STRUCTURE DETERMINATION
Reference: McMurry Ch 13 George et al Ch 3.1, 3.2
The major steps involved in determining the structure of an unknown compound are:
Isolation and Purification
Isolation and purification of an unknown compound is an essential first step to identification. It may be conducted by chemical methods or physical methods
Elemental Analysis
Quantitative analysis for C, H and N in an organic compound is routine and if heteroatoms such as the halogens, S or P are absent, oxygen is usually assumed to make up the difference to 100%.
Example: A compound returns the following analysis: C = 54.55%, H = 9.09%. What is its empirical formula? Assume O = 100 � (54.55 + 9.09) = 36.36%.
Moles of C in 100 g 54.55 / 12.01 = 4.54
Moles of H in 100 g 9.09 / 1.008 = 9.02
Moles of O in 100 g 36.36 / 16.00 = 2.27
This gives a ratio C:H:O of 2:4:1 i.e. an empirical formula of C2H4O
Molecular Formula
In order to determine the molecular formula of a compound, the molecular mass of that compound is required. Benzene (C6H6) and acetylene (C2H2) both have the empirical formula CH, but different molecular masses and molecular formulas.
Mass spectrometry is used to determine the molecular mass of an organic compound. A small sample of the compound is vaporised under very low pressure and high temperature and the vapour is irradiated with a beam of high energy electrons (4000 � 6000 kJ mol-1).
This causes electrons to be ejected from molecules in the sample, leaving them as positively charged cations � the molecular ion or parent ion.
M + e- ® M+. + 2e-
The molecular ions (M+.) may break down into fragment ions or daughter ions (m+).
M+. M+. ® m+ + neutral fragment
Example:

The cations are accelerated through an electric field into a magnetic field where they are deflected. The ions are characterised by their mass (m) to charge (z) ratio (m/z). The charge is always = 1.

The mass spectrum of a compound typically shows a number of signals and the peak at highest m/z (molecular ion) usually corresponds to the mass of the whole molecule. The signals with lower m/z are fragment ions and can provide some structural information.
Example: The mass spectrum of butanone would be predicted to have a signal at the following m/z values:
|
m/z |
Ion |
|
|
72 |
CH3CH2COCH3+ |
Molecular ion or parent ion |
|
57 |
CH3CH2CO+ |
Fragment ions or daughter ions |
|
43 |
CH3CO+ |
|
|
29 |
CH3CH2+ |
Isotopes
Each fragment recorded in the mass spectrum registers the specific isotopes of the various elements present. Some elements have more than one isotope of high natural abundance (e.g. bromine - 79Br 49 % and 81Br 51 %; chlorine - 35Cl 75% and 37Cl 25 %). In these cases, any organic compound that contains, for example, a bromine atom, will appear as two signals separated by two m/z units in the mass spectrum. One signal is associated with ions that contain 79Br and one for the ions that contain 81Br. The relative intensities of these two signals will be approximately the same. Similarly, for any organic compound that contains a chlorine atom, the mass spectrum will contain two signals separated by two m/z units, one for the ions that contain 35Cl and one for the ions that contain 37Cl. In this case, the relative intensities of the two signals will be approximately 3:1, which reflects the natural abundance of 35Cl and 37Cl.
High resolution mass spectrum
In many instances it is not possible to assign a molecular formula to a compound on the basis of the m/z of its parent ion. For example, a parent ion at m/z 72 could be due to C4H8O or C3H4O2 or C3H8N2. If the mass spectrum is recorded with extremely high precision ("high resolution") then the mass of the parent ion, or any fragment, can be recorded to much better than integer precision. Since the mass of the atoms of each element is known to high accuracy, molecules that have the same mass (when it is measured only to the nearest integer mass unit) can usually be distinguished when the mass is measured to 4 decimal places. The accurate masses of 12C, 16O, 14N and 1H are 12.0000 (by definition), 15.9949, 14.0031 and 1.0078 so ions with the formulas C4H8O+, C3H4O2+ or C3H8N2+ would have masses 72.0573, 72.0210 and 72.0686 which can be distinguished by high resolution mass spectroscopy. Note: m/z for the molecular ion (M+) and the molar mass (M) are the same to 4 decimal places since the mass of the electron (the difference) is so small.
FUNCTIONAL GROUPS
Absorption spectroscopy
Organic compounds absorb radiation in different regions of the electromagnetic spectrum. The energy of radiation is proportional to its frequency
(E = hn) and the frequency and wavelength of light are related by the speed of light (l n = c). The absorption of electromagnetic radiation can be detected and used to identify features of the molecule and this is termed absorption spectroscopy.
Ultraviolet-visible spectroscopy
Radiation in the ultraviolet (UV) and visible region of the spectrum has the correct energy to excite electrons in one orbital into an orbital of higher energy. The electrons that are most easily promoted are those in conjugated p-bonds. A conjugated molecule is one in which there is an alternation between single and multiple bonds in at least part of the molecule, for example: aromatic compounds, 1,3-dienes,
(e.g. H2C=CH-CH=CH2), 1,3-diynes, (e.g. HC�C-C�CH) and a,b-unsaturated carbonyl compounds (e.g. propenal, H2C=CH-CHO).

Organic compounds which contain conjugated multiple bonds strongly absorb ultraviolet-visible radiation.
Examples:

Infrared spectroscopy
Electromagnetic radiation in the infrared (IR) region of the spectrum has the correct energy to cause bonds in a molecule to stretch and bend. Individual functional groups have a characteristic absorption in the IR region. The absence of an absorption in the IR spectrum of a compound can be important. For example, if an oxygen-containing compound shows no absorption in the C=O region (1680-1750 cm-1) or in the O-H region (2500 - 3650 cm-1) of the IR spectrum, the compound is likely to be an ether.
|
Bonds |
IR absorption (cm-1) |
|
Bonds |
IR absorption (cm-1) |
|
C-H |
2850-3200 |
|
C� C |
2100-2300 |
|
N-H |
3200-3600 |
|
C� N |
~ 2250 |
|
O-H (alcohol) |
3500-3650 (broad) |
|
C=C |
~ 1600 |
|
O-H (COOH) |
2500-3600 (very broad) |
|
C=O |
1680-1750 (strong) |
The region of an infrared spectrum below about 1500 cm-1 is termed the fingerprint region. Many absorptions in this region result from vibrations of the molecule as a whole and no two compounds have exactly the same absorption in the fingerprint region.
In summary

Nuclear magnetic resonance (NMR) spectroscopy
The nuclei of some isotopes of many elements (e.g. 1H) absorb electromagnetic radiation in the radiofrequency (Rf) region of the spectrum when they are placed in a strong magnetic field. The measurement of the absorption of Rf radiation by nuclei in a magnetic field is called Nuclear Magnetic Resonance spectroscopy.
The number of signals in an NMR spectrum corresponds to the number of distinct types of hydrogen atoms in a molecule. For example, methyl acetate (CH3COOCH3) has two distinct hydrogen environments; the hydrogen atoms of the methyl group directly attached to the oxygen atom are in a different chemical environment to the hydrogen atoms of the methyl group bonded to the carbonyl carbon atom.

The frequency axis of the spectrum is calibrated with a scale (called the d scale) in dimensionless units called parts per million (ppm). The d-scale is calibrated with respect to a reference compound, tetramethylsilane, (CH3)4Si (abbreviated as TMS), taken as d = 0.00 ppm. The frequency difference between a signal arising from a sample and the TMS signal is called the chemical shift.
The chemical shifts of all signals in an NMR spectrum are measured in ppm (d scale) from the TMS signal. The 1H NMR signals from hydrogen atoms in alkyl groups come typically in the region 0 - 2 ppm from TMS; the hydrogen atoms attached to carbon atoms adjacent to carbonyl groups typically occur 2-3 ppm from TMS and the hydrogen atoms attached to aromatic rings occur in the region 7-8 ppm.
Examples:

The hydrogen atoms in most organic molecules give rise to signals in the range 0 to 10 ppm from TMS. The relative intensities of the various signals in a 1H NMR spectrum is proportional to the number of hydrogen atoms that give rise to the signal. For example, the 1H NMR spectrum of CH3-O-CH2-Cl would exhibit two signals with intensities in the ratio 3:2.
The signals in a 1H NMR spectrum frequently show fine structure (i.e. more than one peak) which is termed splitting or multiplicity. The splitting pattern of an NMR signal arises from the hydrogen atoms attached to the neighbouring carbon atoms. If a hydrogen atom has "n" equivalent hydrogen atoms on adjacent carbon atoms, its NMR resonance will appear as a signal which is split into "n+1" lines - this is called the n+1 rule.
|
number of neighbouring hydrogen atoms (n) |
signal multiplicity (no of peaks) |
name of signal |
|
0 1 2 3 4 |
1 2 3 4 5 |
Singlet Doublet Triplet Quartet Quintet |
The NMR signal of a hydrogen nucleus is not split by other hydrogen atoms that are the same as itself. The NMR signal for ethane (CH3-CH3) is a singlet with no splitting because all of the hydrogen nuclei are the same and no hydrogen is split by any of the others. The signal of a hydrogen nucleus is generally only visibly split by other hydrogen nuclei that are no more than three bonds away.
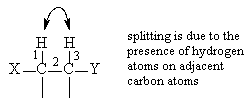
For example, in CH3CH2Br, the 1H NMR spectrum would show two signals of 3:2 intensity, around d 3 and d 1 ppm, with a triplet signal for the CH3 group and a quartet signal for the CH2 group. The signal for the CH3 group appears as a triplet because on the adjacent carbon atom there are 2 hydrogen atoms ("n+1"=2+1=3). The quartet splitting of the CH2 group arises because on the adjacent carbon there are 3 hydrogen atoms ("n+1"=3+1=4).

Quartet signals characteristically have relative line intensities 1:3:3:1; triplets 1:2:1 and doublets are composed of two lines of equal intensity.
In summary
|
Information |
Observation |
|
No. of H environments |
No of signals |
|
Type of H environment |
Position of signal |
|
No of H of each type |
Size of signal |
|
No of adjacent H |
Multiplicity of signal |
The technique is also used in medicine - Magnetic Resonance Imaging (MRI).

