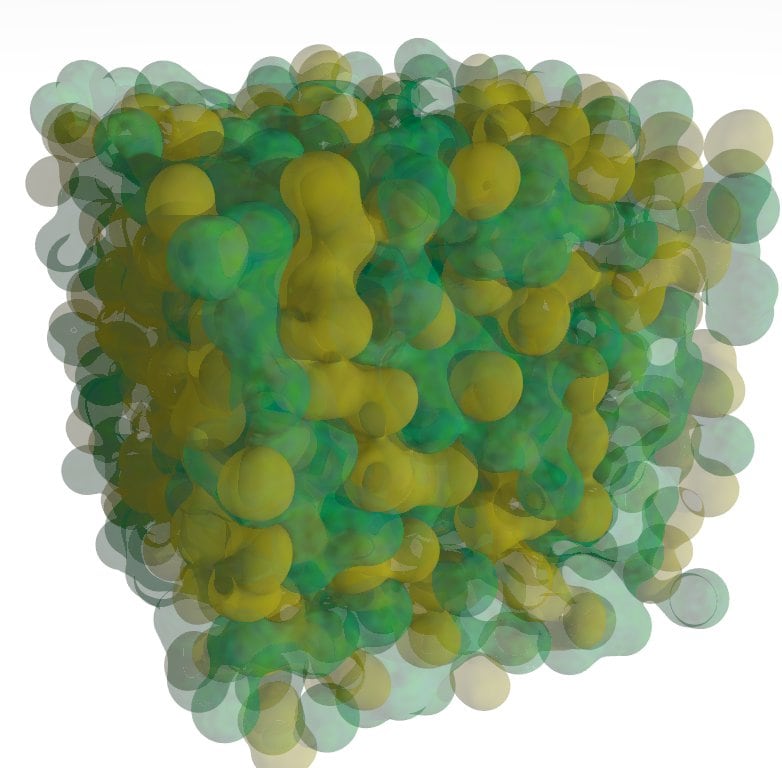
Simulation of the liquid structure of ethylammonium nitrate based on neutron scattering data shows an interpenetrating bicontinuous network of nonpolar (green) and ionic (yellow) functional groups. Are all ILs similarly structured?
SELF-ASSEMBLY IN IONIC LIQUIDS
Ionic liquids (ILs) are salts that melt at low temperatures. E.g. ethylammonium nitrate has a melting point of 13°C. We have recently discovered many room-temperature ionic liquids share with water the ability to promote the self-assembly of surfactants into micelles1, liquid crystals2,3 and microemulsions4. We have also discovered that many ILs themselves behave like surfactants, and organise into sponge like structures in bulk liquid5 (see figure) and oriented layers at surfaces6, 7. The unique properties of ILs such as negligible vapour pressure has led to their wide use as solvents in synthesis and catalysis. How do liquids that are nanostructured themselves affect the interactions between solutes, particles, or surfaces in chemical reactions or self-assembly processes?
In this project we will investigate how changing the structure and H-bonding capacity of IL-forming ions affects the formation, stability and structure of microemulsions. ILs are also very good glass-formers, so we will further examine how IL-microemulsions can be quenched into a solid state to create novel, porous and composite amorphous solids.
- M.U. Araos, G.G. Warr, Langmuir 2008, 24, 9354-9360
- R. Atkin, S.M.C. Bobillier, G.G. Warr J. Phys. Chem. B 2010 , 114 , 1350–1360
- M.U. Araos, G.G. Warr, J. Phys. Chem. B. 2005, 109, 14275-14277
- R. Atkin, G.G. Warr, J. Phys. Chem. B. 2008, 112, 4164-4166
- R. Atkin, G.G. Warr, J. Phys. Chem. B 2007, 111, 9309-9316
- R. Hayes; G.G. Warr; R. Atkin Phys. Chem. Chem. Phys. 2010 12 , 1709-1723.
- R. Atkin and G.G. Warr, J. Phys. Chem. C. 2007,111, 9309-9316