





|
Metal-Organic Frameworks
Coordination frameworks, also referred to as coordination polymers, are crystalline networks of metal ions, or metal ion clusters, coordinatively linked via molecular ligands that contain two or more metal binding sites. The combination of metal ions possessing well defined coordination geometries with an extensive selection of molecular bridging ligands, allows for immense customisation in these frameworks. This can give rise to a number of attractive structure-related properties, including controlling pore environments for gas separation and storage, tuning spin crossover as a function of host-guest interactions, and anomalous thermal expansion behaviour as a result of structural flexibility.
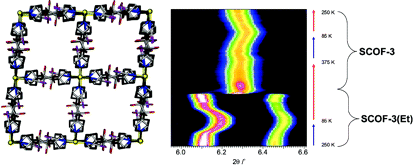
The spin crossover framework SCOF-3 showing a change in the powder diffraction pattern with solvent uptake.
As a consequence of ligand size and bonding directionality, these materials often form frameworks that have the ability to support extensive microporous voids capable of reversible host-guest interactions. These materials may also exist in interpenetrated forms, where one or more identical (yet independent) frameworks fill the available pore space. Differences in synthetic methods can often result in either a single or interpenetrated network. Robson et al. demonstrated that interpenetration could be avoided by creating a charged framework that required the accommodation of additional counterions in order to maintain charge balance. As such, the presence of the bulky counterion within the network cage could prevent interpenetrating networks from forming, thus yielding a single-network material with significant pore space upon substitution of the counterion with a smaller one.
By understanding the factors contributing to a framework's porosity, materials containing some of the largest reported pore volumes have been synthesised. As such, these coordination frameworks have become increasingly popular candidates in gas capture applications, such as hydrogen fuel storage and carbon dioxide capture/catalysis. Whilst large pore volume is a seemingly attractive feature for gas capture applications, studies have found that frameworks with reduced pore dimensions are able to maximise the interaction between the framework and adsorbent, consequently obtaining similar gas uptake values to those of ultra-porous frameworks.
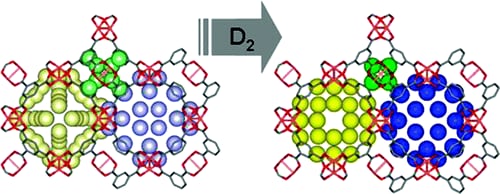
D2 storage in Cu3(benzenetricarboxylate)2.
Ultimately, it is the ability to infinitely and rationally modify the components of coordination frameworks that makes them appealing candidates for use in a variety of technological applications. Therefore, attaining an extensive understanding of the relationship between structure and function will allow for improved rational design, further enhancing their desired functions.
|



