Anomalous Thermal Expansion
The majority of materials expand upon heating, which can have a detrimental effect on sensitive devices where precise alignment is required when working with variable temperatures. Materials that exhibit negative thermal expansion (NTE) can be of great benefit in these devices, such that carefully matched components can result in neither expansion or contraction. Another useful class of meterials, although less common, are those with zero thermal expansion (ZTE). As the name suggests, these materials neither expand nor contract upon heating.
As materials are heated, thermal energy populates vibrational states of the components and of the material as a whole. In most materials, the increased vibration leads to the increase of bond lengths, causing expansion. However, in some materials certain vibrational modes can lead to a decrease in the distance between two groups linked by a vibrating bridge. A common example is the 'skipping rope' vibrational mode in metal cyanide frameworks, such as Prussian Blues.
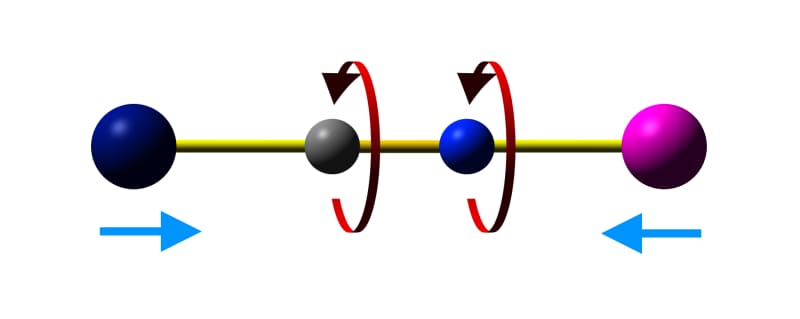
Skipping rope vibrational mode in cyanide-bridged metal frameworks.
Other compounds also show NTE through different vibrational modes in more complex systems, such as MOF-5. A recent publication from our group elucidates the effects of each of the vibrational modes of the benzenedicarboxylate linker and of its interactions with the Zn4O cluster.
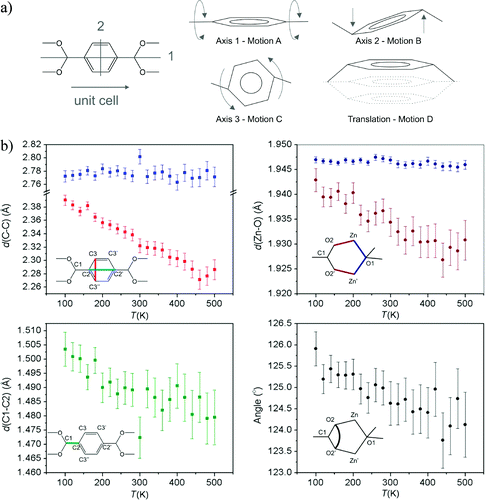
Vibrational modes of MOF-5: a) Sketches of possible simple motions of the benzene ring: rotation around the three principle axes of the benzene ring and a translational up-and-down motion. Axes 1 and 2 are sketched on the left-hand figure. Axis 3 is perpendicular to axes 1 and 2. The translational motion and rotation around axis 2 can be viewed as in-phase and out-of-phase transverse vibrational motions, respectively. b) Temperature-dependent atomic distances and angles derived from the SCXRD data. Upper left: d(C2-C2') (blue squares) and d(C3-C3'') (red squares); lower left: d(C1-C2); upper right: d(Zn-O1) (dark blue circles) and d(Zn-O2) (dark red circles); lower right: O2-C1-O2' angle (black circles).
|



