Trevor Hambley Research Group
Research Areas
Solid tumours account for over 85% of all cancers. Current treatments for solid tumours lack specificity and are often associated with severe side effects. With the aim of improving efficacy of chemotherapy drugs, a number of prodrugs that can exploit the differences between healthy and cancerous tissue are being investigated.
Prodrugs have the potential of remaining inert until they are activated at the target site. Bioinorganic prodrugs can be designed to utilise tumour microenvironments such as hypoxic regions where they can be activated by bioreduction.
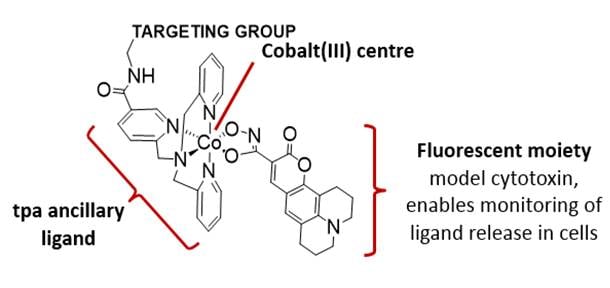
Metal based prodrugs - Cobalt
Kinetically inert Co(III) complexes are reduced to more labile Co(II), resulting in the release and activation of cytotoxins or model cytotoxins in hypoxic regions.
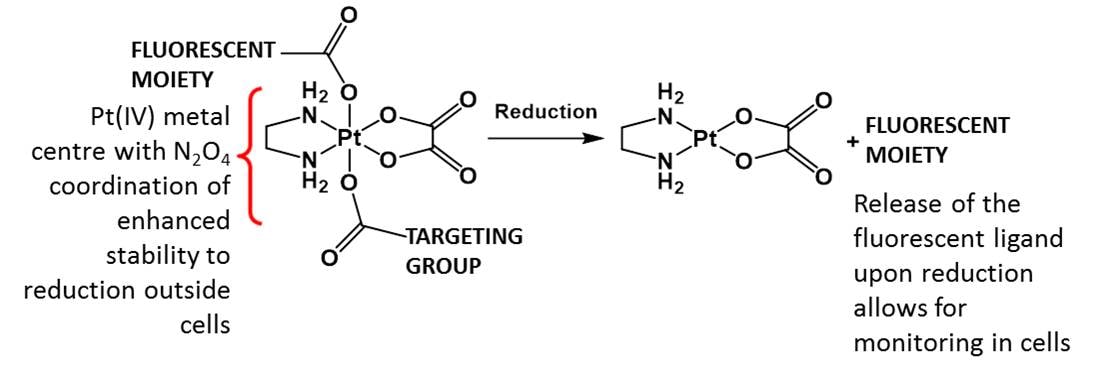
Metal based prodrugs - Platinum
Platinum(IV) prodrugs with diaminetetracarboxylato coordination spheres exhibit unusual resistance to reduction by biological reductants but are easily reduced within cancer cells.
Targeting strategies - targeting cancer cells using glucose
Elevated levels of glucose uptake is one of the hallmarks of malignant cells. Deregulation of the expression of glucose transporters (GLUTs) has been identified in many tumour types, and results in overexpression of these sugar transporters in cancer cells.
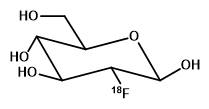
The tumour imaging agent FDG, a radiolabelled glucose analogue, utilises the higher rate of glucose uptake to enable visualisation of tumours and metastases.
Glycoconjugation of metal complexes aims to improve selective delivery and increase uptake of anticancer agents by cancerous cells.
Targeting strategies - targeting prostate cancer by exploiting PSA
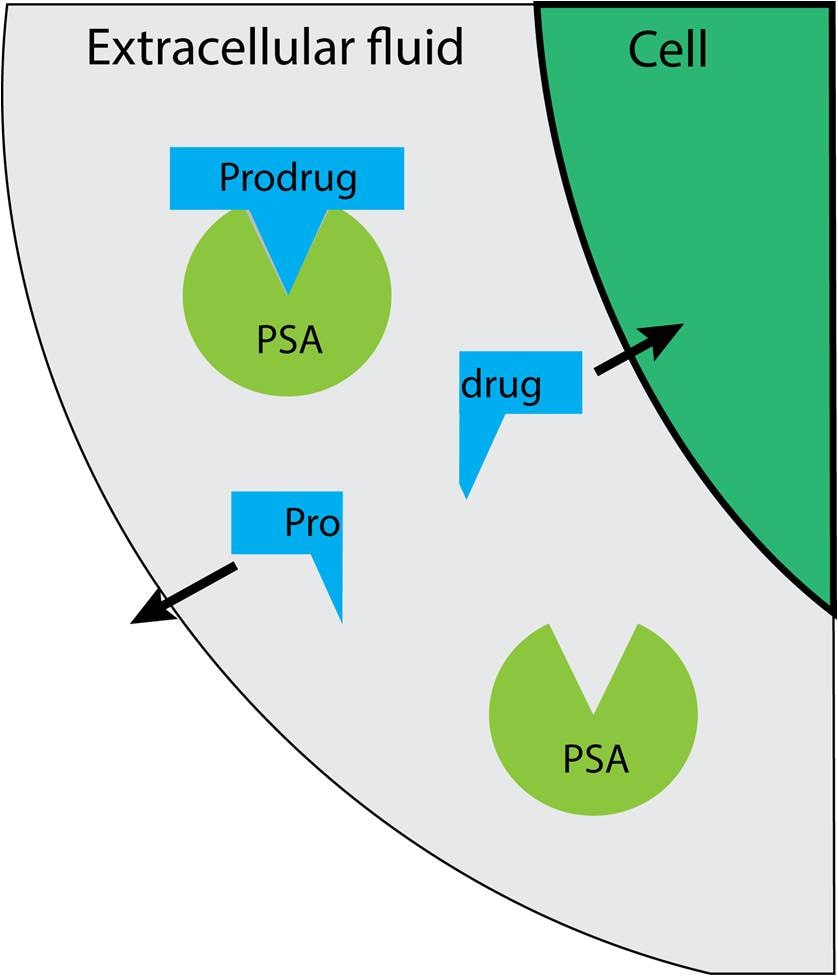
Prostate specific antigen(PSA) is overexpressed by cancerous prostate cells. Active PSA is able to cleave peptide substrates.6,7 Therefore prodrugs containing peptide sequences that are cleaved exclusively by active PSA would be activated in the extracellular fluid of the prostate cell and allow uptake of the activated drug by the cell enabling it to perform its function.
Targeting strategies - targeting cancer cells by exploiting PSMA

Prostate specific membrane antigen (PSMA) is overexpressed by cancerous prostate cells at all stages of prostate cancer. Low molecular weight urea based inhibitors have been shown to selectively bind to PSMA, while quickly clearing non-target organs and have increased tumour penetration.
This type of inhibitor has the potential of delivering the cytotoxin to the target site.
Metal based imaging agents
Radionuclides such as 99mTc can be localised to a tumour site by conjugating the radioisotope to a biological vector that targets the tumour. Non-radioactive Re is an excellent model for 99mTc because they have near-identical coordination preferences. In particular, the tricarbonyl core fac-[M(CO)3]+, where M = 99mTc or Re, is a popular structural core for fluorescent Re complexes.

