The Kassiou Research
Group
Medicinal Chemistry & Drug Discovery
|
Research |
|
Medicinal Chemistry of CNS Active
Molecules We have an extensive medicinal chemistry program
evaluating structure-activity relationships of a number of molecules varying
from polycyclic to heterocyclic scaffolds that interact with specific targets
we think are involved in brain disease. The purpose of these studies is to
identify lead molecules that can be further developed into drug candidates
for the treatment of disease. These projects will involve the synthesis of a
series a compounds that aid in the identification of structural motifs
responsible for optimizing activity. With some examples shown below. Please
inquire for specific details and other projects. |
|
|
|
|
|
|
|
|
Neuroprotective Agents Targeting the
Translocator Protein The translocator protein (TSPO) is ubiquitously
expressed in peripheral tissues but only sparingly in the healthy brain.
Increased levels of TSPO expression have been noted in neuroinflammatory
conditions such as Alzheimer’s disease and Parkinson’s disease coinciding with
activation of microglia. Several
TSPO ligands demonstrate neuroprotective properties in animal models of CNS
disease making the TSPO an important therapeutic target. Within our
group projects involve the discovery and synthesis of novel heterocyclic
chemotypes as new TSPO ligands ii) investigation of their structure-activity
profile ii) evaluation of the functional activity of all newly synthesised
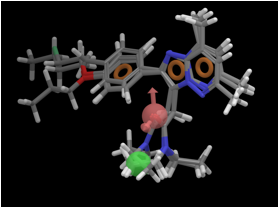
TSPO ligands and their utility as drug candidates. (TSPO pharmacophore hypothesis (AHRRR, where A = hydrogen bond
acceptor, H = hydrophobic group, and R = ring system). Pink sphere – hydrogen
bond acceptor, green sphere – hydrophobic group, tan hoop – ring system. |
|
|
|
|
|
P2X7 Receptor Ligands in
the Treatment of Depression |
|
|
|
Activation of P2X7 receptors (P2X7R)
by ATP has been shown to stimulate the release of interleukin-1b (IL-1b).
Considering that IL-1b can induce behavioural changes that resemble
depression and that P2X7R antagonists play an important role in
modulating IL-1b, it could be hypothesized that blockade of P2X7Rs
might result in antidepressant-like properties. The P2X7R is an
unusual non-desensitising cation selective ion channel directly gated by
extracellular ATP. This is the most interesting of the ionotropic P2X
receptors. Upon stimulation by high concentrations of ATP it generates a
non-selective membrane pore which is permeable to hydrophilic molecules with
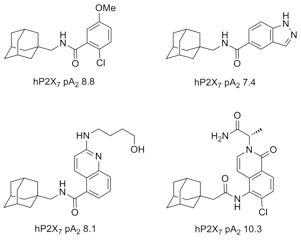
molecular weight up to 900 Da. Recently a series of polycyclic ligands
(examples shown) which displayed potent antagonistic properties at the P2X7
receptor have been reported. Modification of the polycyclic moiety of these
molecules represents new exciting lead structures for development of suitable
antidepressants. |
|
|
|
|
Novel Sigma Receptor Ligands in CNS
Disorders Since the discovery of sigma receptors, research has
been ongoing in an attempt to understand the functional roles of these sites.
Initial interest in sigma receptors was largely motivated by the observation
that the sigma site was a high affinity binding site for psychoactive drugs
including many of the atypical antipsychotic drugs. Ligands which bind with
high affinity at sigma receptors have been shown to modulate and interfere
with several neurotransmitters and have potent activities in animal models suggestive
of antipsychotic, cognitive enhancing, neuroprotective, and antidepressant
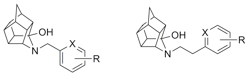
activities. We have recently reported the synthesis and binding a novel
series of trishomocubanes of the type 4-azahexacyclododecane which display
high affinity for sigma receptor subtypes. We are currently refining SAR in
order to develop higher affinity ligands suitable for in vivo studies of
sigma receptor pharmacology.
|
|
|
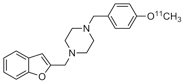
Arylalkyl 4-benzyl piperazines have only recently
been developed as radioligands for imaging sigma receptors. This receptor is
of interest since many psychoactive drugs including atypical antipsychotics
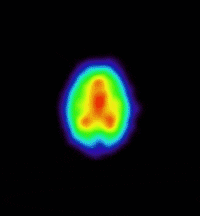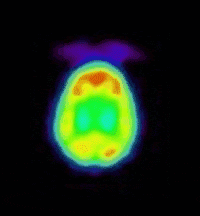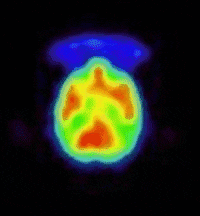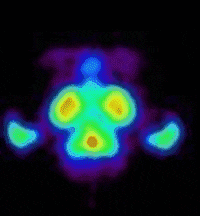
bind to this site. The prototypic radioligand, 1-benzofuran-2-ylmethyl-4-(4-[11C]methoxy-benzyl)piperazine, has been evaluated in the
living baboon brain (see images) and has demonstrated in vivo sigma receptor
binding. This projects aims at exploring the structure activity profile of
the arylalkyl 4-benzyl piperazine scaffold in the development of subtype
selective sigma radioligands for imaging using PET.
(Animation showing the binding of a sigma
receptor ligand in the living baboon brain.) |
|
![]()