Decoded stretchy molecule gives living tissues flexibility
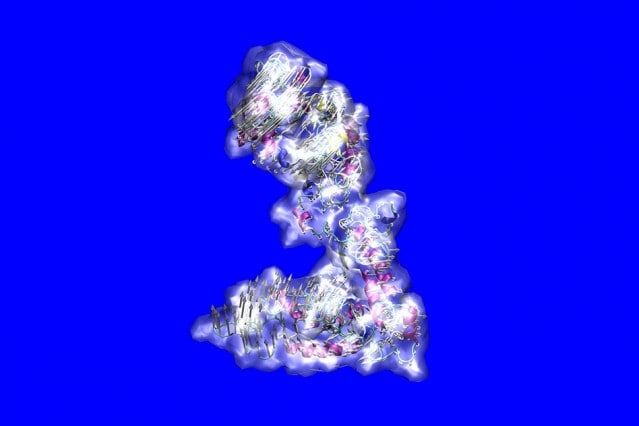
The configuration of the tropoelastin molecule, which forms the basis for the elastin that gives tissues like skin and blood vessels their elasticity. Supplied by the researchers.
The stretchiness that allows living tissues to expand, contract, stretch, and bend throughout a lifetime is the result of a protein molecule called tropoelastin. Remarkably, this molecule can stretch to eight times its length, always returning to its original size.
Tropoelastin is the precursor molecule of elastin which, along with structures called microfibrils, is the key to flexibility of tissues including skin, lungs, and blood vessels. But the molecule is complex, made up of 698 amino acids in sequence and filled with disordered regions. Unravelling its structure has been a major challenge.
Using a combination of molecular modeling and experimental observation to build up an atom-by-atom picture of the molecule’s structure, researchers from the University of Sydney, MIT and University of Manchester have now decoded the molecular structure of this complex molecule.
Published this week in the Proceedings of the National Academy of Sciences, the research also identifies what can go wrong with its structure in various genetically driven diseases
Author and postdoctoral researcher Anna Tarakanova, from the School of Engineering at Massachusetts Institute of Technology (MIT), said the structure of tropoelastin had been elusive until now.
“Traditional characterisation methods are insufficient for decoding this molecule because it’s very large, disordered and dynamic,” she said.
“However the combination of computer modeling and experimental observations used allowed us to predict a fully atomistic structure of the molecule.”
The study showed how certain disease-causing mutations in the gene that controls the formation of tropoelastin change the molecule’s stiffness and dynamic responses, which could help in the design of treatments or countermeasures for these conditions.
‘Artificial’ mutations induced by the researchers can be used to better understand the function of the specific part of the gene affected by that mutation. The study also looked at the specific changes in the tropoelastin molecule caused by mutations associated with known diseases, such as cutis laxa, in which the skin lacks elasticity and hangs loosely.
Co-author Professor Anthony Weiss, the University of Sydney’s McCaughey Chair in Biochemistry, from the Faculty of Science and multidisciplinary Charles Perkins Centre, said the techniques used to unravel the structure of topoelastin were powerful.
This superb global collaboration shows we can now use computers to predict the performance of human elastic molecules.
“It also helps us to accelerate new ways to repair damaged tissue, like that in wounds,” Professor Weiss added.
Co-author Markus Buehler, the Jerry McAfee Professor in Engineering and Head of the Department of Civil and Environmental Engineering at MIT, said the findings had implications beyond disease.
“Understanding the structure of this molecule is not only important in the context of disease, but can also enable us to translate the knowledge from this biomaterial to synthetic polymers, which can be designed to meet certain engineering needs,” he said.
The research team also included Dr Giselle Yeo, an Early Career Development Fellow from the University of Sydney’s Charles Perkins Centre and School of Life and Environmental Sciences; Professor Clair Baldock from the University of Manchester in the UK. The work was supported by the National Institutes of Health, the Office of Naval Research, the National Science Foundation, the Australian Research Council and the Wellcome Trust.
