
Under-12s are increasingly catching COVID-19. How sick are they getting and when will we be able to vaccinate them?
In July Australia’s Therapeutic Goods Administration (TGA) provisionally approved the Pfizer COVID-19 vaccine for kids aged 12-15.
The Australian Technical Advisory group on Immunisation (ATAGI) subsequently recommended kids in this age group with underlying chronic medical conditions, Aboriginal and Torres Strait Islander children and kids living in remote communities should be prioritised.
We’re expecting advice from ATAGI as to whether the rollout should be extended to all 12 to 15-year-olds, as countries like the United States and Canada have done.
But where does that leave children under 12? We know they’re making up a large proportion of new infections in Australia’s current outbreaks, which was not the case last year.
Do they need to be vaccinated? What are the benefits of vaccinating children, both for the child and the community? And how will we know the vaccines are safe and effective for young children?
COVID in kids
Throughout the pandemic, fortunately, we’ve seen children are very unlikely to get severely unwell or die from COVID-19.
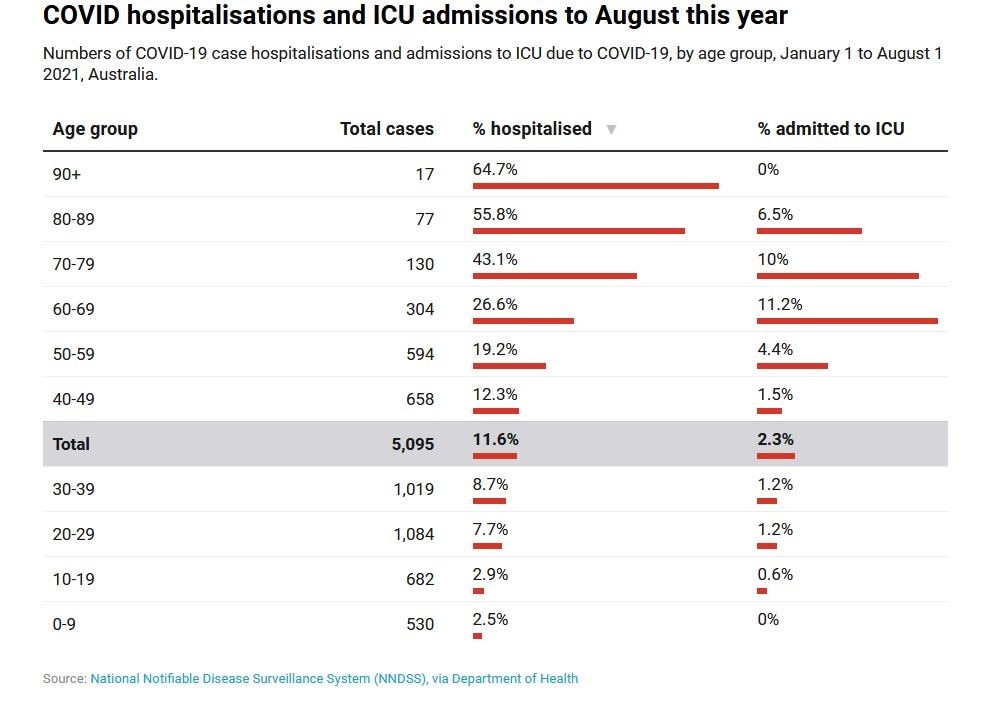
Australian data from January 1 to August 1 this year show 2.5% of children aged up to nine and 2.9% children and teenagers aged 10-19 who contracted COVID were hospitalised. This is compared to 7.7% of young adults aged 20-29, with the rates continuing to increase with age.
Cases are on the rise among children in New South Wales, but to date this hasn’t been accompanied by a large increase in paediatric hospitalisations.
Recent data show increased rates of hospitalisation among children in the US with COVID-19 compared to last year, alongside rising infections with the Delta variant.
But even though the rate has gone up, it remains low. In children and adolescents aged 17 and under the rate is 0.38 per 100,000 people, well below the rate in adults aged 60 to 69 (5.63 per 100,000) and those over 70 (8.07 per 100,000).
However, some kids who have chronic medical conditions are at a higher risk of getting really sick from COVID, which is why ATAGI has listed them as a priority group.
One of the complications of COVID-19 is long COVID where a person experiences lasting symptoms such as breathlessness, anxiety and “brain fog” (reductions in attention and concentration).
Reassuringly, a recent study found only a small proportion of children had symptoms beyond four weeks after their initial COVID infection, and almost all children had recovered by eight weeks.
So what are the benefits of vaccinating kids?
While the Delta variant is more infectious than other strains of the coronavirus, and more kids are becoming infected, there’s not a scientific consensus at this stage that it’s causing more severe disease in children.
That said, a small minority will get sicker than others and need hospital care.
If vaccines are found to be safe and effective for younger children there would be benefit in protecting the individual child.
What about collective benefits? Will vaccinating young children reduce transmission in the community and improve our herd protection?
Recent modelling from the Doherty Institute doesn’t appear to consider whether vaccinating children under 12 would or wouldn’t contribute to reducing community transmission.
Other modelling has suggested vaccinating younger children and adolescents will be important if Australia is to reach the elusive “herd immunity”.
Trials are under way
Clinical trials of the mRNA vaccines from Pfizer and Moderna in children aged 12 and up have shown good results (though at this stage Moderna is only approved in Australia for adults).
Before we move to vaccinating children under 12 we’ll need safety and efficacy data from trials in this age group.
It’s important to conduct clinical trials specifically in children because their immune systems are different. For example, children may experience different side effects following vaccination, and may need a smaller dose.
Trials of the COVID-19 mRNA vaccines in younger children are under way. The Moderna trial KidCOVE is currently recruiting in the US. So far close to 7,000 kids are enrolled.
Meanwhile, Pfizer is aiming to enrol 4,500 children under 12 across the US and other countries.
The studies are divided into children aged six to 11, aged two to five, and six months to less than two years old. They are aiming to assess safety and immune responses after two vaccinations with three different dose sizes.
For Pfizer, the three doses being trialled are 10 micrograms, 20 micrograms, and 30 micrograms (the latter is the dose given to older teens and adults).
A trial of AstraZeneca’s COVID-19 vaccine in children aged 6-17 commenced in March 2021 in the United Kingdom. However this trial was paused as a precautionary measure following reports of blood clots in adults who received this vaccine.
What now?
You may be wondering when children under 12 will be able to be vaccinated. The short answer is we don’t know for sure.
We need strong safety and efficacy data from the clinical trials before considering vaccinating young children. Currently, it’s anticipated the first data on children younger than 12 may be available for review later this year.
For now though, it’s reassuring to know children still appear less likely to end up in hospital with COVID compared to adults.
Further, it seems many of the cases we’re seeing in children are due to transmission in the household, often from an infected adult to the child.
So the best way to protect younger kids for now is to ensure as many adults as possible are fully vaccinated.
High vaccine coverage in the community will also benefit children by reducing the need for lockdowns and school closures, which we know can have negative effects on their education, socialisation and mental health.
This article was first published on The Conversation and is written by Associate Professor Nick Wood from the University of Sydney.